Halia Therapeutics Pipeline
Pipeline
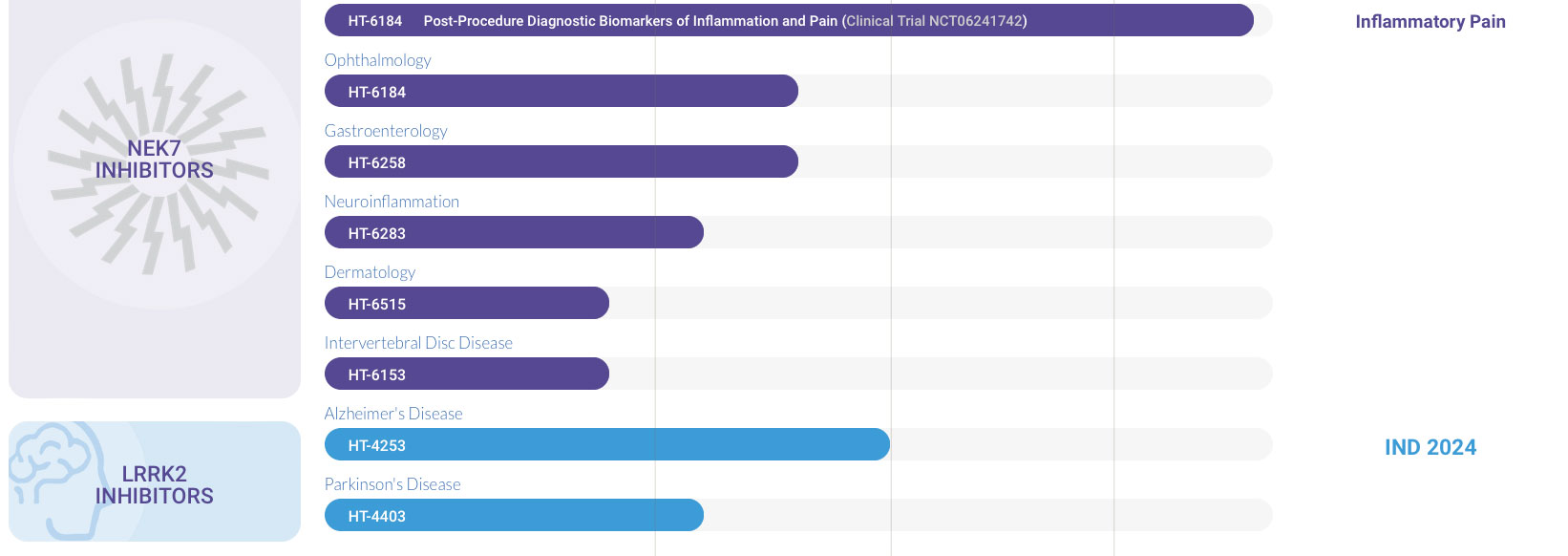
NEK7
INHIBITORS
LRRK2
INHIBITORS
Discovery | INDEnabling | Phase 1 | Phase 2 |
- Oral Systemic Multiple Indications 98%
- 98%
- Ophthalmology 50%
- Gastroenterology 50%
- Neuroinflammation 40%
- Dermatology 30%
- Intervertebral Disc Disease 30%
- Alzheimer's Disease 59.6%
- Parkinson's Disease 40%
HT-6184 Lower-Risk Myelodysplastic Syndromes (LR-MDS) (Clinical Trial CTRI/2023/11/059758)
HT-6184 Post-Procedure Diagnostic Biomarkers of Inflammation and Pain (Clinical Trial NCT06241742)
HT-6184
HT-6258
HT-6283
HT-6515
HT-6153
HT-4253
HT-4403
MDS
Inflammatory Pain
IND 2024
Halia Therapeutics Policy for Expanded Access to Investigational Drugs
Halia is committed to developing novel meaningful medicines for patients with serious diseases who may benefit from new treatment options. Halia’s current policy for evaluating and responding to requests for individual patient access to investigational drugs intended to treat serious diseases is found below.
Our product pipeline consists of investigational drugs currently being tested in clinical trials and have not yet been approved by the US Food and Drug Administration (FDA). Since the FDA has not approved these investigational drugs, we do not have significant clinical findings demonstrating their safety and effectiveness. Halia’s investigational drugs are currently at a stage in development where we are focused on enrolling patients in our clinical trials and continuing to learn more about our investigational drugs’ safety and efficacy. We encourage patients to speak with their physicians about their eligibility for enrollment in any of Halia’s clinical trials whenever possible. Our clinical trials are designed, conducted, and monitored to advance the development of our investigational drugs to make them more broadly available to patients in the future. Additional information about Halia’s ongoing clinical trials is available at https://clinicaltrials.gov using the search term “Halia.”
Currently, Halia cannot make expanded access available to its investigational drugs.
Halia may revise this expanded access policy at any time in the future, and this posting will be updated should there be any policy change.
For more information regarding our investigational drugs or questions about participation in one of our clinical trials, please submit a request to info@haliatherapeutics.com. Halia personnel will acknowledge receipt of any expanded access questions within ten business days of receipt.
For additional information on expanded access to investigational drugs, visit the FDA’s website.
Contact Us
Corporate Headquarters
3900 N. Traverse Mountain Blvd., Suite 100
Lehi, Utah 84043
United States
info@haliatx.com | +1 (385) 355-4315